Permeation is the molecular penetration of gases , vapours, or fluids through the material membrane of solids.
Permeation rates are inversely proportional to subsurface concentration and are a function of membrane thickness, intrinsic permeability, and mass diffusivity property of the material. Permeability for any substance is temperature dependent, but not pressure dependent.
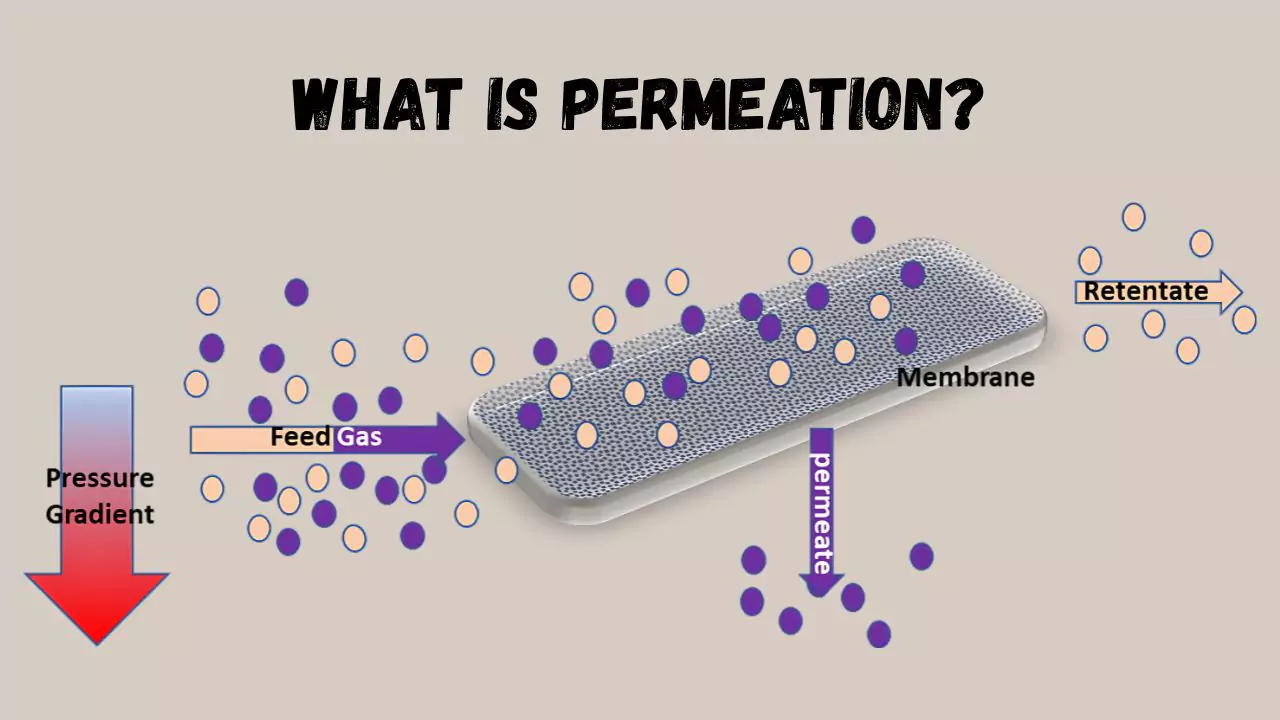
How Permeation Works
Permeation is when molecules of the permeant have diffused through a membrane or interface. Permeation has to do with the diffusion of the permeant across the interface, and there is a concentration gradient from high to low.
When the permeant molecules encounter the interface an additional process happens to allow the full process of permeation to occur. The transient presence of the permeant at the interface is either absorbed or desorbed according to the process of sorption.
In a thin membrane, there is a constant permeation flux that is only governed by the charge crossing the entry face, while in thick membranes the diffusion flux is inversely proportional to the subsurface concentration.
Permeation can occur in through most materials, such as ceramics, polymers, and metals; although metals have a lower permeability than ceramics or polymers due to the nature of porous crystal structure.
Conversely, polymers have a high rate of permeability. There can also be semipermeable materials where there are only certain molecules or ions, with particular properties, can diffuse through the membrane.
Permeation metals can result in embrittlement and blistering, while permeation through coatings occurs from the diffusion and solubility of water (or other fluid) can, in turn, cause substrate corrosion.
Permeation is dependent on the temperature of the permeation between two components, as well as the attributes of the permeant, and material being permeated.
Permeation Measurement
There are a number of ways to measure the permeation of a material. These ways measure the permeability of a substance through a particular material.
In metals, sensors are utilized to measure the permeation. The sensors establish the permeation current density and weight loss or weight gain of the substrate using a wet and dry cycle experiment. The permeability coefficient is proportional to the concentration of the diffusing substance(s), and inversely proportional to the solubility coefficient.
Films and membranes can use any gas or liquid to measure permeation, with one such method employing a central module partitioned by a test film. The method of testing gas is fed up one side of the cell, while a sweep gas collects the permeated gas, transporting it to detector.
Another method of testing is by intermittent contacting, in which a sample of the test chemical is placed onto the surface of the material permeability is to be measured. Then adding or removing some specific amounts of the test chemical, the permeability material is analysed for concentration of the test chemical in the structure.
By comparing the time that the test chemical had contact with the material it is possible to calculate cumulative permeation of the test chemical in the material. Permeation is modeled by equations including Fick’s laws of diffusion, and it can be measured using a minipermeameter.
Permeation due to diffusion is expressed in SI units which are mol/(m・s・Pa), but Barrers are often used as well.
Uses for Permeation
The uses of permeation are extensive, and probably familiar to both industry and in the home. Permeation is used in gas separation, and it is instrumental in determining the effects that hydrogen has on high strength steel in the petroleum and petrochemical industries.
Hydrogen permeation can have corrosive, electrochemical, or surface reaction concerns plus cathodic protection, which can cause cracks from the accumulation of hydrogen in the bulk of an alloy, from high stress and consequently from trapping. These cracks can affect the integrity of a structure.
Permeation is important for thermoplastic and thermoset pipes for carrying water under pressure from potentially huge leaks by permeation, which would be taken to be failure, if you can measure permeation of water through pipe wall.
Permeation is very important for insulation materials, when you think of submarine cables, where you want to be able to eliminate the vapor permeation of water so that you can protect the conductor from corrosion.
If permeation resistance is important for insulating materials, then it is also important for clothing i.e. determining breakthrough times and chemical protective properties for clothing to be used in situations in industry, such as in the nuclear industry.
In the case of tyres, it is preferable to avoid permeation, as dramatically as possible, i.e. air pressure in the tyres should be preserved to maintain driving safety. Over time, gas will permeate irrespective of the material used, but the best material should allow the lowest rate of gas to escape from the tyre.
Selective permeation is sometimes desirable, such in responding to a potential need for different sealing techniques in packaging. Some packages would require hermetic seals, others may not need a hermetic seal but the selective permeation is desirable, so understanding permeation, and the particulars of packaging, is important.
Another example of use for the medical industry is drug delivery, specifically drug patch delivery.
The drug patches are polymer based and contain a chemical reservoir that is released to the essential constituent patient treatment like drugs can be passed to the patient as contact activates the permeation process through the polymer membrane according to the concentration gradient established.
Because the chemical reservoir was over loaded the drug prolongs and unintentional burst and lag mechanism rates, where the chemical would swing to transferring very high rates, as the patch is in contact with the skin but as soon as the concentration gradient is established it oscillates to a sustained rate.
Despite the accidental burst and lag process being likely not utilized with drug absorption there is major significance to delivery as uses such as in the Ocusert System.
Will, in other instances, minimize permeation across the membranes to meet pharmaceutical and batch production like that of injection drugs both for eliminating the other substances entering into pharmaceutical product, and or halting the product from being lost to evaporating.
Often the chemical-containing ampoules would be made from low permeation materials including glass or synthetic materials.