What is Graphite?
Graphite, once known as plumbag, is a fascinating, naturally occurring form of crystalline carbon. Its structure is distinctly hexagonal, giving it properties that are both unique and practical. You’ll often find graphite as a native element mineral, nestled within metamorphic and igneous rocks, and it’s rightly described as a mineral of extremes.
This mineral forms deep beneath the Earth’s surface when carbon experiences intense heat and pressure, specifically, conditions reaching around 75,000 pounds per square inch and temperatures near 750 degrees Celsius.
These conditions correspond to what geologists call the granulite metamorphic facies. Given even greater pressures and temperatures, graphite can actually transform into diamond, which is quite remarkable when you think about the connection between the two.
Visually, graphite presents itself in shades of gray to black, with an opaque appearance and a striking metallic luster. Although it’s flexible, it doesn’t have much elasticity. What makes graphite particularly interesting is its blend of metallic and non-metallic traits.
On the one hand, it conducts heat and electricity very well, features we typically associate with metals. On the other hand, it also demonstrates non-metallic qualities, such as chemical inertness, high thermal resistance, and a slippery feel (what scientists refer to as lubricity).
In everyday life, you’ll most likely encounter graphite in the form of pencil “lead,” though its uses extend much further. Thanks to its impressive conductivity, graphite is also found in products like electrodes, batteries, and even solar panels.
Its role as a lubricant is another practical application, taking advantage of those non-metallic properties. All in all, graphite is a material whose versatility stems from its unique structure and the conditions under which it forms.

Related Posts: What are Metal and Non-Metal?
Who discovered graphite?
Edward G. Acheson’s discovery of synthetic graphite happened more by chance than by design. While conducting high-temperature experiments with carborundum, he observed that, at approximately 4,150°C (7,500°F), the silicon in carborundum vaporized, leaving behind carbon in the form of graphite. This serendipitous finding led Acheson to secure a patent for the process in 1896, paving the way for commercial production the following year.
By 1918, the industry had shifted toward using petroleum coke as the primary raw material. This substance, composed of tiny and irregular graphite crystals embedded within organic matter, became the standard feedstock for manufacturing graphite with a purity level ranging from 99 to 99.5 percent.

What color is graphite?
Graphite, a naturally occurring form of carbon, is most often recognized by its gray to black appearance. Alongside diamond and amorphous carbon, it stands as one of the three allotropes of carbon.
The specific shade of graphite can shift, largely depending on its level of purity and any impurities it might contain. When graphite is pure, you will generally see it as a deep gray or black. However, when other minerals or contaminants are present, the color may lighten noticeably.
Interestingly, graphite that finds its way into high-temperature settings like furnace linings can sometimes take on a pink or reddish hue, again thanks to particular impurities.
So, while graphite is most commonly identified by its gray or black tones, its exact color is really determined by how pure it is and what else it contains.
Structure of Graphite
Graphite is characterized by its distinctive layered structure, where each layer is made up of six carbon atoms forming hexagonal rings. These layers are set apart from one another, creating widely spaced horizontal sheets.
Because of this arrangement, graphite takes on a hexagonal crystalline form, which is notably different from diamond—even though both are made of pure carbon. While diamond crystallizes in either an octahedral or tetrahedral pattern, graphite’s layers stack neatly in the hexagonal system.
Looking a bit closer, each layer in graphite is a network of carbon atoms connected at the edges, creating a continuous pattern of six-membered rings. This structure can actually be thought of as a limitless array of benzene rings, only without the hydrogen atoms. The result is a stable, extended sheet that explains many of graphite’s unique properties.
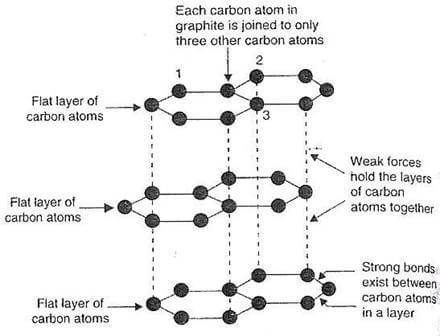
In these ring structures, carbon atoms exhibit sp² hybridization. Under the sp² molecular orbital framework, each carbon forms bonds with three neighboring atoms, specifically, with three other carbons in the structure of graphite. This bonding results in bond angles of 120 degrees between adjacent carbon atoms.
The rings themselves are organized into extensive layers, commonly referred to as graphene layers. Within each layer, the carbon-carbon bond measures approximately 1.418 Å in length.
These graphene layers are stacked parallel to the crystallographic “C” axis, following the hexagonal (four-axis) system characteristic of graphite crystals. If you’re interested in exploring the structure of graphite further, feel free to consult our detailed guide.
Properties of Graphite
Graphite is an allotrope of carbon that is used for making moderator rods in nuclear power plants. Its properties are as follows:
- A greyish-black, opaque substance.
- Lighter than diamond, smooth and slippery to the touch.
- A good conductor of electricity (Due to the presence of free electrons) and a good conductor of heat.
- A crystalline solid
- Very soapy to the touch.
- Non-inflammable.
- Soft due to weak Vander wall forces.
- The conductor of electricity.
physical properties of graphite
Graphite shares a notably high melting point with diamond. Achieving its melting point involves more than simply separating the layers; the robust covalent bonds running throughout its structure must be broken.
In contrast to diamond’s hardness, graphite feels soft and slippery. This property explains its widespread use in pencils and as a dry lubricant, such as for locks.
Imagine a stack of cards: while each card is sturdy on its own, they easily slide past each other or even detach from the pile. Similarly, when you write with a pencil, thin sheets of graphite slide away and transfer onto the paper.
Graphite is also less dense than diamond. The reason lies in its structure—spaces between the layers account for the lower density, as these gaps are essentially “unused” compared to the closely packed arrangement in diamond.
Much like diamond, graphite does not dissolve in water or organic solvents. The attraction between solvent molecules and carbon atoms is simply not enough to disrupt the strong covalent bonds that hold the graphite together.
One distinct characteristic of graphite is its ability to conduct electricity. The presence of delocalized electrons within the layers allows them to move freely. When graphite becomes part of an electric circuit, electrons can flow off one end and be replaced by incoming electrons at the other, enabling electrical conductivity.
Chemical properties of Graphite
| Color | Iron-black to steel-gray; deep blue in transmitted light |
| Chemical Classification | Native element |
| Streak | Black |
| Luster | Metallic, sometimes earthy |
| Diaphaneity | Opaque |
| Cleavage | Perfect in one direction |
| Mohs Hardness | 1 to 2 |
| Specific Gravity | 2.1 to 2.3 |
| Diagnostic Properties | Color, streak, slippery feel, specific gravity |
| Chemical Composition | C |
| Crystal System | Hexagonal |
Types of Graphite
The Different Types of Graphite:
- Natural Graphite
- High Crystalline graphite
- Amorphous graphite
- Flake graphite
- Synthetic Graphite.
1. Natural Graphite
Natural graphite occurs as a mineral, presenting itself in various degrees of crystallinity. Commercially, most graphite is obtained through mining, and it typically comes mixed with other minerals. Once extracted, the raw graphite must undergo significant processing. Froth flotation is a common method to increase its concentration and purity.
One of the defining qualities of natural graphite is its remarkable ability to conduct heat and electricity, a property that holds steady even across a wide range of temperatures. It is also notable for its impressive strength and its high melting point, which is around 3650 °C.
Because of these characteristics, natural graphite finds its way into a number of important applications, such as refractory materials, batteries, steel production, expanded graphite, brake linings, foundry facings, and lubricants.
When it comes to commercial availability, natural graphite is typically classified into three distinct forms, each derived from naturally occurring sources. Each of these types brings its own set of properties, making it especially suitable for specific uses within industry.
1.1 Crystalline Graphite
Crystalline vein, sometimes called lump graphite, stands out as the rarest and most sought-after type of natural graphite, prized for its exceptional quality.
Unlike other forms, this graphite originates through the direct deposition of solid graphitic carbon from high-temperature subsurface fluids, crude oil being a notable example, which are gradually transformed into graphite under the influence of prolonged heat, pressure, and time.
At present, Sri Lanka remains the sole region in the world actively producing crystalline vein graphite. This material is particularly valued in industry because it is remarkably easy to machine; it can be shaped into solid components without the need for binder additives, offering a practical and cost-effective alternative to lower-grade graphite sources.
Typically, these graphite veins measure anywhere from 1 centimeter up to 1 meter in thickness, and even before any refining process, their carbon content usually exceeds 90%, often reaching between 95% and 99%.
Thanks to this naturally high purity, vein graphite can serve many of the same purposes as flake graphite but often delivers a clear competitive edge, both in pricing and in its suitability for specialized applications, including lubricants, battery production, grinding wheels, and powder metallurgy.
1.2 Amorphous Graphite
Amorphous graphite typically forms as a result of contact metamorphism, where an anthracite coal seam interacts with a metamorphic agent. This process produces microcrystalline graphite, often referred to as amorphous graphite in the field.
Among the different types of natural graphite, amorphous graphite is considered the least abundant. Its graphite content can range quite widely, from 25% up to 85%, largely influenced by the specific geological conditions present during its formation.
Under the microscope, amorphous graphite appears as very fine, crystal-like particles embedded in mesomorphic rocks such as coal and slate. After refining, its carbon purity remains relatively modest, generally falling between 70% and 85%. Without magnification, these tiny particles are not readily visible to the naked eye.
Because of its lower purity and fine grain size, amorphous graphite is primarily used in applications where high-quality graphite is not essential. It finds its way into products like pencils, lubricants, refractories, paints, metallurgical materials, coatings, brake pads, and as an additive in rubber manufacturing.
Economically, it is the most affordable form of graphite. Major deposits of amorphous graphite are located in countries such as China, Mexico, and the United States.
1.3 Flake Graphite
Natural flake graphite originates when carbon-based material undergoes intense pressure and elevated temperatures. While the initial carbon may be sourced from either organic or inorganic matter, most commercially produced flake graphite actually comes from organic deposits. Generally, the formation process demands pressures exceeding 1 gigapascal and temperatures above 750°C.
Within metamorphic rocks, flake graphite appears either evenly distributed throughout the ore body or clustered in distinct, lens-shaped concentrations. The carbon content across these deposits can range quite a bit, typically falling between 5% and 40%. Often, you’ll encounter flaky graphite in a flaky or lamellar form, especially in rocks like limestone, gneiss, and schist.
To separate flake graphite from its host rock, foam flotation is commonly employed. Through this process, “floating” graphite can reach concentrations between 80% and 90%. If higher purity is needed, further chemical processing is used, allowing for graphite with a purity exceeding 98%. Notably, these valuable graphite deposits are scattered across the globe, making flake graphite a resource with broad geographic distribution.
2. Synthetic Graphite
Synthetic graphite is typically produced from coke and pitch, although the resulting material does not reach the same level of crystallinity as natural graphite. There are specialized forms, however, such as highly ordered pyrolytic graphite (HOPG), which is characterized by an angular misalignment of less than 1° between its graphite layers.
Broadly speaking, synthetic graphite can be divided into two main categories. The first is electro graphite, a form of pure carbon that results from processing coal tar pitch and calcined petroleum coke in an electric furnace. The second type is synthetic graphite generated by subjecting calcined petroleum pitch to extremely high temperatures, reaching around 2800 °C.
In comparison to natural graphite, synthetic varieties tend to have greater electrical resistance and increased porosity, coupled with a lower density. This higher porosity is a notable disadvantage when it comes to refractory applications, where a denser material is often necessary.
Chemically, synthetic graphite consists mainly of graphitic carbon, achieved through graphitization, the heat treatment of non-graphitic carbon sources, or via chemical vapor deposition from hydrocarbons at temperatures above 2100 K.
As for where synthetic graphite finds its use, its applications span a range of fields, including aerospace components, carbon brushes, graphite electrodes, batteries, and moderator rods within nuclear power plants. That said, its high porosity is precisely why it is not preferred for use in refractories.
Uses of Graphite
Graphite has been used since ancient times. It has a wide range of applications in the modern world too.
Let’s look at some common uses of graphite below:
- Writing Materials
- Lubricants
- Refractory
- Nuclear Reactors
- Batteries
- Graphene Sheets
1. Writing Materials
The term “graphite” has its origins in the Greek word meaning “to write.” Unsurprisingly, one of graphite’s most familiar uses is in the production of pencil leads. Interestingly, the “lead” in pencils isn’t pure graphite; instead, it’s a blend of clay and graphite in its amorphous state.
2. Lubricants/Repellents
Graphite serves as a key component in the formulation of lubricants, such as grease. When exposed to atmospheric moisture, graphite undergoes a reaction that leads to the formation of a thin film on the applied surface.
This layer effectively lowers friction, making it valuable in various mechanical contexts. Beyond lubrication, graphite finds application in the manufacture of car brakes and clutch systems.
In its powdered lump form, graphite is also incorporated into certain types of paint. The reasoning behind this use stems from graphite’s inherent resistance to water. When added to paint, it imparts a protective quality, shielding wood and similar surfaces from moisture and wear.
3. Refractories
Because of its remarkable resistance to heat and chemical stability, graphite serves as a key refractory material across various industries. Its properties make it especially valuable in manufacturing settings, where it plays a central role in producing glass and steel, as well as in iron processing.
4. Nuclear Reactors
Graphite has the capacity to absorb fast-moving neutrons, which plays a crucial role in stabilizing nuclear reactions within reactors. For this reason, it is commonly employed as a moderator in nuclear facilities to help maintain control over the reaction process.
5. Electrical Industry
Crystalline flake graphite finds essential applications in producing carbon electrodes, brushes, and plates, all of which are integral components of dry cell batteries and various electrical devices. Notably, natural graphite is often further refined to create synthetic graphite, a material particularly valuable for its role in lithium-ion battery technology.
6. Graphene Sheets
Graphite serves as a source material for producing graphene sheets, which are recognized for being approximately one hundred times stronger and ten times lighter than steel.
This remarkable form of graphite has found use in the manufacture of sports equipment, valued for its combination of lightness and strength.
Looking ahead, many researchers and industry professionals are exploring potential applications for graphene in areas such as medicine and aerospace, given its unique set of properties.
FAQs
What is graphite used for?
Graphite is used in pencils, lubricants, crucibles, foundry facings, polishes, brushes for electric motors, and cores of nuclear reactors.
Its high thermal and electrical conductivity make it a key part of steelmaking, where it is used as electrodes in electric arc furnaces.
In the early 21st century, global demand for graphite has increased because of its use as the anode in lithium-ion batteries for electric vehicles.
Is graphite 100% carbon?
Graphite is one of only two naturally occurring forms of pure carbon, the other being diamonds. Graphite occurs in a two dimensional, planar molecular structure whereas diamonds have a three dimensional crystal structure.
Graphite generally occurs as flakes, which are multiple layers of graphene held together by weak bonds. Graphene is a single, one atom thick layer of carbon atoms arranged in a “honeycomb” or “chicken wire” pattern.
Is graphite a stone or a metal?
Graphite is a non-metal but has many properties of metals. It is an excellent conductor of heat and electricity and has the highest natural strength and stiffness of any material.
It maintains its strength and stability to temperatures in excess of 3,600°C and is very resistant to chemical attack. At the same time it is one of the lightest of all reinforcing agents and has high natural lubricity.
Is graphite a rust?
Graphite is one of the most inert natural materials known. It will resist corrosive attack by a wide variety of chemicals including many acids, bases, solvents, oils, metals, etc.
Graphite is corrosion-resistant against most common acids (e.g. hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. It has limited resistance against oxidizing media (e.g. nitric acid) and bases (e.g. amines, potash and caustic soda).
Why is graphite so valuable?
It boasts unique properties such as high electrical conductivity, resistance to heat, and the ability to maintain its structural integrity under extreme conditions.
Graphite finds application in various industrial sectors, including aerospace, automotive, electronics, and construction.
Synthetic and natural graphite are consumed on a large scale (1.3 million metric tons per year in 2023) for uses in pencils, lubricants, and electrodes.
Under high pressures and temperatures it converts to diamond. It is a good (but not excellent) conductor of both heat and electricity.