Common Metal Identification Methods
Identifying the metal one has can be important for many processes for example, welding, machining, cutting and fabrication.
There are a number of methods that may be used to identify a piece of metal by a way of field identification. Some of the common methods of identification are a tests of surface appearance, spark test, chip test, magnet test and sometimes a hardness test. In some cases you can simple identify a metal from its surface appearance.
Metalworkers use many forms of identification, both traditional and modern ones, to identify the scraps and sheets of metals that come into the shop. In this post, we will be discussing some known traditional and modern metal identification methods and the benefits and drawbacks of those methods.
Traditional Testing Method
Some of the common traditional testing methods are Appearance, Spark, Rockwell and Brinell Hardness. The main advantage to these tests is the cost savings, but also the drawbacks are the heavy reliance on personnel experience and methods that may also destroy the samples.
1. Appearance Test
The appearance tests might not always yield sufficient information, but it might be enough to classify the metal. The appearance tests considers the color of the metal and whether there is a machined mark (or no machined mark) on the surfaces of the metal.
2. Spark Test
To perform a spark test, you would bring a piece of metal into contact with the fast portable or fixed grinder with sufficient pressure for a spark of the spark stream to be emitted.
An experienced metal worker intuitively observes the spark stream visually to determine the metals and considers the length, color and shape of the spark stream before identifying the metal.
With this visual spark testing technique we would recommend allowing this test for experienced technicians only.
3. Rockwell Test
In order to carry out this test, a Rockwell hardness testing machine is required. The intent of the method is to quantify the depth of an indentation formed by a cone-shaped point in the testing machine.
This test is limited in the sense that it only shows one of many metal properties – hardness of the metal. Soft metals will have deeper impressions and hard metals will have lighter impressions.
4. Brinell Hardness Test
The Brinell hardness test is like the Rockwell hardness test because both tests measure the metal indentation made by a controlled object. However, the Brinell hardness test measures the area of the indentation.
A hardened ball is pressed into the metal surface under a load of 3000 kg, which makes an indentation. The area of the indentation is measured and assigned a hardness number. The larger the indentation area indicates a softer metal leading to a lower hardness number.
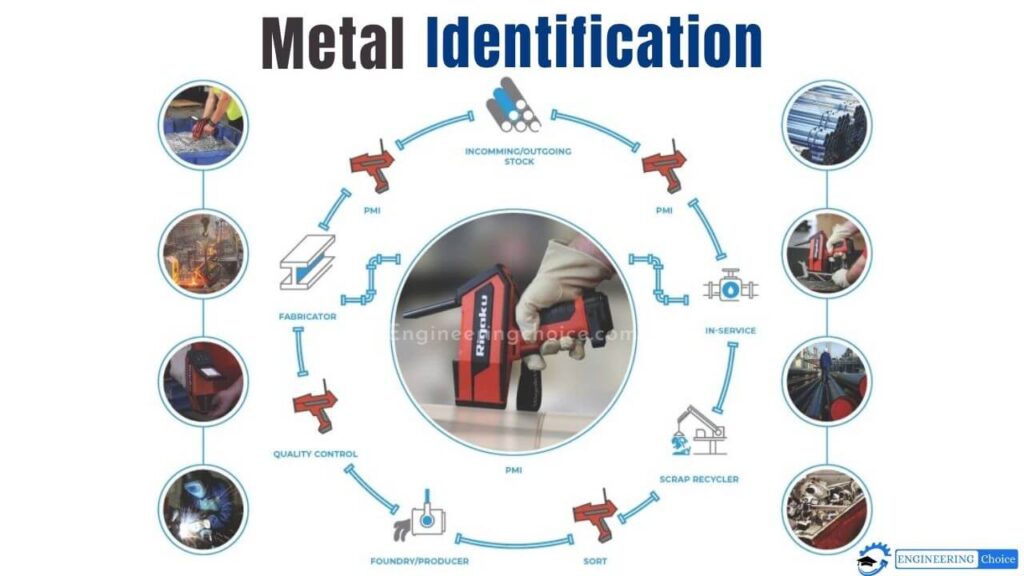
Modern Metal Testing Methods
No longer dependent on just the human eye or experience, current methods of metal testing utilize various technological processes to accurately and rapidly identify metals and protect the samples.
One method is named Positive Metal Identification (PMI) which utilizes X-ray fluorescence (XRF) and Optical Emission Spectrometry (OES). PMI is the testing of a metallic alloy to determine its constituent parts and to identify the appropriate grade of alloy by reading (percentages) of the elements.
PMI analyzers provide detailed analyses of the elements of the materials for purposes ranging from industrial uses to research.
XRF and OES techniques are both prevalent in the asset recovery industry for their accuracy, as well as, providing results only seconds after testing. There are minor differences in the two techniques and the differences will be shared below.
1. Optical Emission Spectrometry
Optical Emission Spectrometry (OES), is user-friendly, rapid, and it can indicate the precise quantitative breakdown of solid materials.
OES, also referred to as Atomic Emission Spectrometry, applies the intensity of the light emitted at a given wavelength to identify the elemental makeup of a sample. Like fingerprints, the rays and emitted light are unique to types of metal.
An analysis will be presented as a percentage breakdown. OES analysis can be applied in stationary, portable, or mobile formats. Taken together with its rapid nature, versatility, and user-friendly nature, it is the ideal alloy testing method.
2. X-Ray Fluorescence
X-Ray Fluorescence (XRF) is a very precise and accurate quantitative method to measure the elemental composition of materials. XRF spectrometers use high energy X-rays to stimulate samples into producing specific characteristic rays that are then registered by the XRF spectrometer.
XRF has specific requirements, and you will need a handheld XRF gun, but it is a very rapid reading process that can occur in fractions of a second. The metals detected at high percentage levels can take a few seconds to read, while metals at part-per-million levels can take up to a few minutes. Either way, there is no faster reading.
3. X-Ray Diffraction
X-Ray Diffraction (XRD) is a technique utilized to provide chemical composition data of metals. Often, XRD is used with XRF as XRD is a step further in assessing and providing context.
XRD works by determining the crystalline phases present and then compares those identified crystalline phases to a database of archived phases. XRD analyzes the purity levels in ground powder.
XRD is useful for analyzing minerals, polymers, corrosive products, and many unknowns. XRD is useful to analyze, quantify, and provide texture data regarding phases.
Unlike traditional methods of analyzing metals that take years to train, metal workers that are proficient in the PMI technique can be skillfully trained to go into a unique metal analysis job in minutes.
4. Laser-Induced Breakdown Spectrometer (Libs)
A laser-induced breakdown spectrometer (LIBS) is also distinguishable from atomic emission spectrometry for the fact that it uses a highly energetic laser pulse to excite the sample. While generally non-destructive, this method is gaining traction and so is increasingly useful for scrap metal analysis.
Visually Identifying Common Metals
Ferrous or Nonferrous?
Ferrous materials contain iron, which often means they are magnetic, whereas non-ferrous materials do not contain iron. Examples of ferrous materials include mild steel (referred to as low-carbon steel) and examples of non-ferrous materials include copper and aluminum. It is always advisable to take a magnet to the scrap yard.
Aluminum
Aluminum is a shiny gray metal and has a clear oxide that forms when in contact with air – not exactly the best thing for identification, but the melting point for aluminum is 658° C (1217°F). Aluminium is also non-sparking.
Density of aluminium is 2.70 g/cm3, which is a good way of identifying it as you can get density of the material easily because density = mass ÷ volume. As I mentioned previously, aluminium is non-ferrous.
Bronze
Most bronzes are an alloy of copper and tin but architectural bronze actually contains a trace amount of lead. bronze has a dark coppery color and will get a green oxide over time.
The melting point of bronze is 850-1000°C (1562-1832°F) depending on the amount of each metal. Bronze is nonferrous in nature. Since bronze is an alloy, the density varies. Bronze will vibrate like a bell when struck.
Brass
Another copper alloy is brass, but it has zinc instead of tin, which gives brass a yellow-gold color. The melting point of Brass is 900-940°C (1652-1724°F) depending on the amount of metal used. Brass is also nonferrous.
As an alloy, brass has variable density. Brass vibrates like a bell when you strike it which can help you decipher if something is brass not gold.
Chromium
Chromium has a very shiny silver color forms a clear oxide over time. The Chromium melting point is 1615 °C (3034 °F). You will rarely find things made of pure chromium, but you will find things that have a coating of chromium to make shiny and not so rusty. Chromium density is 7.2 g/cm3. Chromium is nonferrous.
Copper
Copper is manufactured into many different alloys including brass and bronze. Copper’s color is light red and it will develop a green oxide over time. Copper is nonferrous. Copper melts at 1083°C (1981°F). The density of copper is 8.94 g/cm3. Copper, similarly to brass, will vibrate like a bell when struck.
Gold
Gold is a shiny yellow color with no oxide. The melting temperature of gold is 1064.18°C (1947.52°F). Gold is soft and very heavy.
Gold has high electrical conductivity (meaning electricity can pass through it more) which is why many cords have gold plating on the connectors. Gold has 19.30 g/cm3 density. Gold is nonferrous. Gold is a “precious” metal meaning it is very expensive, and used in coins and jewelry.
Iron
Iron contains ferrous (finally!) and magnetic. Iron is a dull grey when unpolished, while its rust is a reddish color. Iron is also in a lot of alloys like steel. Irons melting temperature is 1530°C (2786°F). Irons density is 7.87 g/cm3.
Lead
Lead is a dull-grey color if not polished but is relatively shiny if polished. Lead has a relatively low melting point temperature at 327°C (621°F). Lead is nonferrous. Leads are very heavy with a density of 10.6 g/cm3.
Magnesium
Magnesium has a grayish-white color and develops an oxide that can give it a dull look. Magnesium melts at a temperature of 650 °C (1202 °F). Magnesium easily burns too when in the powder form or small strips of it.
Burning magnesium burns very brightly and is hard to extinguish because it burns so hot, if you were to pour water on it, it breaks the water down into hydrogen and oxygen, both flammable gases!
Mg can also burn without flame (without oxygen), which also makes it harder to extinguish. The magnesium metal has a much better specific gravity (light) being =1.738 g/cm3.
This lightness is one reason magnesium is popular with car engine blocks and the bright burning makes magnesium more desirable for incendiary weapons (to burn stuff up) and fireworks.
Mild Steel
Mild steel’s colour is black or dark grey unpolished and silvery polished. Mild steel has red rust oxide, the same as iron. Mild steel is ferrous and magnetic. Mild steel also has an alternative name which is low-carbon steel.
Mild steel makes yellow sparks when ground down. Mild steel density is approximately 7.86 g/cm3 that can vary, since it is an alloy of iron and carbon (low carbon steel). The mild steel melting point is 1350-1530°C (2462-2786°F).
Nickel
Nickel is shiny silver when polished and otherwise darker colored at some stages. Nickel is somewhat unique in being a non-iron alloy, magnetic metal (the U.S. nickel is made with a copper-nickel alloy and is not magnetic). Nickel has a melting point of 1452 °C (2645 °F). Nickel has a density of 8.902 g/cm3.
Stainless Steel
Stainless steel is characterized by a shiny silver color that does not oxidize. Chromium is blended in with steel, and when it hardens, the chromium leaves a coating of oxide on top of the steel, this oxide is too thin to see through and therefore the steel shows through.
Stainless steel melting point is from 1400-1450 °C (2552-2642 °F). The density of stainless steel varies because it is an alloy. The magnetic characteristic of various stainless steels depends on the alloy (ie some are magnetic) but all are ferrous.
Tin
When polished, tin is a silver color (like most metals), but it is darker when it is not polished. Tin has a relatively low melting point of 231°C (449°F). Tin has a density of 7.365 g/cm3. Tin is nonferrous.
Titanium
Titanium is a silvery-grey metal when it is not polished, and it is a darker grey when it is unpolished. Titanium produces bright white sparks when it is ground. Titanium is classified as a nonferrous metal. The melting point of titanium is 1795°C (3263°F). Titanium has a density of 4.506 g/cm3.
Silver
Silver is a bright gray color even before it is polished, but it changes to a black film over the years, and must be polished. Silver has a melting point of 961.78°C (1763.2°F). Silver has the highest electrical conductivity (meaning more electricity can move through it), than any other metal.
Silvers density is 10.49 g/cm^3. Silver is nonferrous. Silver is a “precious” metal so it is expensive, and it is generally used in coins and jewelry.
Zinc
Zinc is naturally dull grey and it’s difficult to polish. Zinc has an oxide that, when it flakes off, takes some of the zinc with it so other things are coated in it, so the zinc “rusts” instead of the base metal, which is called galvanization.
Due to its inexpensive cost, zinc is the predominant metal in us pennies. Zincs melting point is 419°C (786°F). Zinc is in the nonferrous classification of metals. Zinc density is measured at 7.14 g/cm3 .
FAQs
How can I tell what metal I have?
Use a magnet: a magnet should be your first step because it will help determine whether a metal is ferrous or non-ferrous. Feel the weight: stainless steel and aluminum are both shiny and can be mistaken for each other, but stainless steel is heavier. Determine hardness: using a metal file, scrape the metal.
Is there a tool to identify metals?
PMI analyzers provide detailed element analysis of materials for uses from industrial to research. Both XRF and OES techniques are widely used in the industry because they provide accurate results within seconds of testing.
How can you identify a metal based on its appearance?
The most basic test is done by observing the metal’s appearance. Though not always as accurate as most tests a trained/experienced metal worker can identify metals by characteristics such as the color and texture of their machined and unmachined surfaces.
What are the numbers for metal identification?
The SAE system is based on the use of four-or five digit numbers. The first number indicates the type of alloy used; for example, 1 indicates a carbon steel. Two indicates nickel steel. The second, and sometimes the third, number gives the amount of the main alloy in whole percentage numbers.
How to test metals at home?
Magnet Test: Perhaps the simplest of the tests, the magnet test determines whether or not a metal is magnetic by holding it to a magnet. It’s worth noting, however, that some stainless steels are not magnetic, so it’s not always a foolproof test on its own.