What is Galvanization?
Galvanization is a process where a protective zinc coating is applied to steel or iron to keep rust at bay. The most widespread technique is hot-dip galvanizing, which involves dipping metal parts into a bath of molten zinc so that they come out with a full, even layer of protection.
This method is hugely popular because it offers a reliable defense against corrosion. Essentially, a thinner layer of zinc shields a much thicker base metal underneath, helping the metal last longer in harsh conditions.
If you take a moment to look around when you’re out driving, you’ll notice plenty of examples: street signs, guardrails, and lampposts often have that dull silver finish. What you’re seeing is the zinc coating at work, quietly extending the life of the steel below.
There are some real advantages to using galvanized steel, especially when it comes to construction and repairs. Thanks to the zinc coating, galvanized steel tends to require less maintenance, which translates into lower long-term costs.
Without some form of protection, steel naturally starts to rust when exposed to air and moisture. The amount of rust depends a lot on where the steel is kept. Rust, for those curious, forms when iron reacts with oxygen and water—resulting in that familiar reddish iron oxide.
Now, while painting or using a plastic coating can help, these approaches have their drawbacks. If either layer gets scratched or damaged, rust quickly sneaks in and starts spreading, causing the protective coating to peel off. In the end, these methods often fall short in terms of lasting protection.
Hot-dip galvanizing, on the other hand, does a more thorough job. By dipping the entire piece in molten zinc, every surface gets completely covered. Zinc corrodes much more slowly than iron or steel, which means the base metal stays protected for much longer—making hot-dip galvanizing a dependable choice for safeguarding steel.

How Does Galvanization Protect the Base Metal Underneath?
- Zinc Coating as a Barrier: When a metal is coated with zinc, it’s essentially shielded from corrosive elements think of things like acid rain or other aggressive chemicals. This outer layer acts as a protective barrier, stopping those substances from ever reaching the metal beneath.
- Galvanization and Rust Protection: Galvanization is a tried-and-true method to guard against rust. Even if the zinc layer gets scratched or damaged, zinc still takes one for the team. It’s more “willing” to give up electrons (as an anode) than the metal underneath. So, instead of the base metal rusting, the zinc corrodes first essentially sacrificing itself to keep the metal intact.
- Zinc’s Sacrificial Role (with a Twist): Here’s something interesting: zinc doesn’t just protect by being a barrier—it actually corrodes faster than the metal it’s covering. Sometimes, a bit of chromate is added to the mix. This ingredient speeds up the zinc’s corrosion, which might sound counterintuitive at first. But in reality, it means the zinc layer will “wear out” before the base metal does, continuing to shield the underlying metal from rust for as long as possible.
Why Galvanize?
Galvanizing a metal primarily serves to protect it against corrosion. If this protective layer of zinc were absent, the underlying metal would be vulnerable to environmental factors, making it prone to oxidation and more rapid deterioration.
Using galvanized steel is a cost-effective way to guard against corrosion, especially compared to more expensive options like austenitic stainless steel or aluminum.
How Does Galvanization Work?
Galvanizing offers metals a robust defense against various forms of damage, primarily by adding a protective layer that keeps out harmful environmental factors.
When steel is coated with zinc, this barrier does a solid job of blocking water, moisture, and other potentially corrosive substances in the air. Even so, if something scratches the zinc layer deep enough to expose the steel beneath, that bare spot becomes vulnerable to rust and corrosion.
But galvanizing doesn’t just work as a simple shield. It also involves a process called “galvanic corrosion protection.” This kicks in when two metals with different electrochemical properties touch each other in the presence of an electrolyte—think of salty water as a classic example. At that point, one metal acts as the anode and the other as the cathode.
Here’s where things get interesting: the anode corrodes faster, while the cathode resists corrosion. Zinc is a popular choice for this process because it tends to become the anode when paired with other metals. This means that, when the zinc coating is in direct contact with the underlying steel, the zinc will sacrifice itself (corrode first), keeping the steel (the cathode) protected for a much longer period.
Different Methods of Galvanizing
There are several different processes for galvanizing metal:
1. Hot-Dip Galvanizing
Hot-dip galvanizing is a technique where iron or steel parts are immersed in molten zinc, forming a tough, corrosion-resistant coating made up of zinc-iron alloy and pure zinc layers.
When steel meets that hot bath of zinc, there’s an immediate reaction at the surface—the zinc and iron start to interact at a molecular level, which is really just a fancy way of saying the two metals diffuse into each other to create a new, protective barrier.
One interesting aspect of this diffusion process is that the coating grows evenly in all directions, no matter the shape of the piece. This means every nook and cranny ends up with a consistent layer of protection, not just the easy-to-reach surfaces.
Despite its roots dating all the way back to 1742, hot-dip galvanizing hasn’t stood still. The basic idea—protecting steel by dipping it in zinc—remains, but the techniques and chemistry involved have kept evolving.
What’s remained unchanged, though, is its reputation for providing decades of reliable, maintenance-free corrosion resistance at a cost that’s generally pretty reasonable.
When you break down the process, it’s actually quite straightforward. There are three main steps: preparing the surface, dipping the part in molten zinc, and then a final post-treatment phase. The simplicity here isn’t just a quirk; it’s a real advantage compared to other methods of shielding steel from corrosion.
It’s hard to overstate just how much damage rust and corrosion can do—not just to private property, but to public infrastructure as well. The costs of repairing roads, bridges, or buildings that have been eaten away by rust add up quickly. Without proper protection, the expenses don’t stop at repairs; sometimes, entire structures need to be rebuilt from scratch.
Given the growing focus on sustainability, it’s increasingly important to think long-term. Building things that last, with minimal need for ongoing maintenance, pays off not only for the environment but for the economy, too. Hot-dip galvanizing, with its lasting durability, fits perfectly into this mindset.
2. Pre-galvanizing
This method actually shares a lot in common with hot-dip galvanizing, but there’s a key difference: pre-galvanizing happens right in the steel mill, typically on materials that already have a specific shape.
Here, sheet metal goes through a cleaning routine that’s almost identical to what you’d see in hot-dip galvanizing. After that, the metal takes a dip in a bath of molten zinc, and once it’s coated, it’s pulled out and allowed to cool.
One of the main perks of pre-galvanizing is speed and uniformity—large coils of sheet steel can be coated much faster, and the resulting finish tends to be more consistent than what you get with traditional hot-dip galvanizing. However, there’s a notable downside: as soon as the pre-galvanized metal is cut during later manufacturing, the newly exposed edges end up uncoated and unprotected.
So, when a long coil of pre-galvanized sheet metal gets sliced down to size, those fresh-cut edges are left bare, which can leave them vulnerable unless they’re treated further.
3. Electrogalvanizing
Electroplating stands apart from older methods in that it doesn’t rely on immersing materials in a bath of molten zinc. Instead, it utilizes an electrolyte solution and an electric current to drive zinc ions onto the surface of the base metal.
During this process, the zinc ions, which carry a positive charge, are reduced and deposited as metallic zinc onto the substrate—this substrate acts as the positively charged electrode.
To achieve a consistently smooth zinc layer, grain refiners can be added to the solution. Much like what’s done in pre-galvanizing, electro-galvanizing is often carried out continuously, especially when treating long rolls of sheet metal.
There are a few notable benefits with this approach. It allows for a very uniform coating and gives manufacturers fine control over the thickness of the zinc layer.
That said, the zinc layer produced through electroplating tends to be thinner than what you’d get from hot-dip galvanizing, which means it might not offer the same level of corrosion protection.
What is Hot Dip Galvanizing? In Detail
Hot-dip galvanizing, often referred to as HDG, is a straightforward yet highly effective method for protecting steel from corrosion. The process itself involves immersing the steel in a bath of molten zinc, which coats the metal and provides a durable barrier. Typically, there are three main stages in this process: preparing the surface, carrying out the actual galvanizing, and finally, conducting a thorough inspection.
To break it down a bit further, hot-dip galvanizing falls under the broader category of galvanizing techniques. The essential idea is to take iron or steel and dip it into zinc heated to around 450°C (about 842°F). At this high temperature, the zinc forms an alloy with the surface of the steel, creating a robust and long-lasting protective layer.
Once the steel is out in the open, exposed to air, the zinc layer gets to work. It first reacts with oxygen, forming zinc oxide. This compound doesn’t stop there—it goes on to react with carbon dioxide in the atmosphere, producing zinc carbonate.
This final layer is usually a muted gray and might look a bit dull, but don’t be fooled; it’s actually tough and acts as a shield, preventing further corrosion of the steel underneath.
You’ll often find galvanized steel in places where there’s a real need for rust resistance, but the high price tag of stainless steel isn’t justified. Not only does it strike a good balance between cost and durability, but it also stands out visually; if you look closely, you can spot galvanized steel by the unique crystallization patterns—sometimes called “spangle”—on its surface.
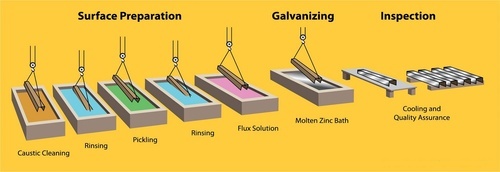
1. Surface Preparation
When steel components reach the galvanizing facility, they’re either suspended by wires or set onto a shelving system, both of which are designed to be moved efficiently through the various stages using overhead cranes. The steel doesn’t go straight to galvanizing; instead, it first undergoes a thorough three-step cleaning process: degreasing, pickling, and fluxing.
Degreasing is all about getting rid of surface grime any dirt, oil, or leftover organic materials that might be clinging to the steel. Once that’s out of the way, the steel is submerged in an acidic pickling bath.
This step targets tougher impurities, specifically mill scale and iron oxide, which can interfere with later stages. The last cleaning phase is called fluxing. Here, any residual oxides are removed, and the steel gets coated with a protective layer. This layer is important because it keeps new oxides from forming before the steel is actually galvanized.
Getting the surface perfectly clean really matters in this whole process. Zinc won’t bond properly with steel if there’s any contamination, so each step in the preparation routine is essential for a successful, durable galvanized finish.
2. Galvanizing
Once the steel surface has been properly prepared, it is carefully dipped into a molten zinc bath maintained at approximately 830°F. The bath typically contains at least 98% pure zinc.
To ensure thorough coating, the steel is lowered into the kettle at an angle, which allows any trapped air especially in tubular sections or awkward pockets to escape. This technique makes sure that the molten zinc is able to flow freely, fully covering and penetrating every part of the steel piece.
As soon as the steel is submerged, a metallurgical reaction occurs between the iron in the steel and the surrounding zinc. This interaction results in the formation of multiple zinc-iron intermetallic layers, topped off by an exterior layer composed entirely of pure zinc.
3. Inspection
The last stage in the process involves closely examining the coating itself. Often, a careful visual inspection is enough to give a reliable sense of the coating’s quality, mainly because zinc simply won’t bond with steel that hasn’t been properly cleaned any missed spots stand out right away as bare, uncoated patches.
On top of that, it’s a good idea to use a magnetic thickness gauge to make sure the coating meets the required specifications for thickness.
Benefits of Hot-dip Galvanizing
Hot-dip galvanizing brings several significant benefits to the steel it protects. One of its key strengths lies in the way the zinc-iron alloy layers bond directly to the steel, creating a robust shield against the environment while also delivering cathodic protection.
In simple terms, the zinc coating doesn’t just sit on the surface—it actively defends the steel beneath it by sacrificing itself if corrosion tries to take hold.
What really stands out about galvanized coatings is their toughness. These coatings are strongly attached to the steel, with a bond strength of roughly 3,600 psi, making them impressively resistant to abrasion.
The intermetallic layers that develop during the process are actually harder than the steel itself. Even if the coating gets scratched or damaged, zinc’s sacrificial properties continue to protect any exposed steel, sometimes up to a quarter-inch away from the damaged area.
Beyond this sacrificial protection, hot-dip galvanizing has other qualities that make it so effective. The way the coating forms is especially important. As the steel is dipped into the molten zinc, a diffusion reaction takes place.
This causes the coating to grow outward from the surface, ensuring that even corners and edges receive as much protection as the flatter sections. Full immersion in the zinc bath guarantees that every part of the steel, including the insides of hollow pieces, gets completely coated.
Another interesting aspect is the natural development of the so-called zinc patina on the surface over time. This patina acts as a tough, impermeable barrier against corrosion. Altogether—the patina, the sacrificial action of zinc, the thorough coverage, and the durability of the coating—these factors explain why hot-dip galvanized steel is known for its long-lasting, maintenance-free performance.
How long does the galvanizing process take?
Usually, the immersion process lasts about four to five minutes, although this duration might increase for heavier items with greater thermal inertia, or in situations where deeper zinc penetration is required, such as with intricate internal surfaces.
After the item is lifted from the galvanizing bath, some molten zinc remains on top of the alloy layer. As this coating cools, it solidifies and gives galvanized products their signature bright, shiny finish.
Post-treatment
After galvanizing, the material can be cooled either by quenching it in water or simply letting it cool in the open air. Interestingly, factors like temperature, humidity, or even the air quality inside the galvanizing facility don’t actually impact the quality of the galvanized coating itself.
This is quite different from the requirements for painting, where those same environmental conditions become very important for achieving a good finish.
Once the galvanizing is complete, there’s generally no need for any additional treatment unless you want to enhance the appearance or add extra protection—say, in settings with particularly harsh exposure. In those cases, applying a layer of paint or a powder coating is a common approach.
If you’re looking to prevent those annoying wet storage stains, using chemical conversion coatings or other types of barrier systems can be really effective. These extra steps aren’t always necessary, but they can make a noticeable difference when conditions call for it.
How Can You Use Galvanized Metal?
Galvanized metals play a significant role in our daily lives, turning up in places we might not even notice at first glance. Take the automotive and cycling industries, for example many car bodies and bicycles rely on galvanized metals for their durability.
Even today, you’ll find certain drinking water pipes made from galvanized steel, not to mention the widespread use of galvanized cold-rolled sheet metal in various manufacturing processes.
Small but essential items like nuts, bolts, tools, and all sorts of wires are commonly galvanized as well. This isn’t just about saving money, although the process is cost-effective; it’s about extending the lifespan of these materials, making them last longer even in tough conditions.
When it comes to construction, galvanized steel stands out, especially in modern steel frame buildings. You’ll also see it featured in balconies, verandahs, staircases, ladders, and walkways—the kinds of structures that need both strength and resistance to the elements.
If you’re working on a project destined for the outdoors, galvanized metal is a solid option. It’s a popular pick for fences, roofing, and outdoor walkways, largely because it can handle whatever the weather throws at it without giving in to rust or corrosion.
Benefits of Galvanizing Metal
- Lower Initial Cost: When it comes to up-front expenses, galvanizing is often more affordable than many other popular protective coatings for steel. This is partly because labor-intensive coatings, like paint, have become much more expensive to apply—whereas galvanizing is a factory-based process that hasn’t seen the same price hikes.
- Minimal Maintenance and Lowest Lifetime Cost: Even in situations where galvanizing costs a bit more at the start, it almost always wins out in the long run. That’s because it lasts longer and doesn’t demand much upkeep. Maintenance becomes especially tricky—and expensive—if the structure is in a hard-to-reach area, or if shutting down production for repairs isn’t really an option.
- Long-Lasting Protection: A properly applied galvanized coating can protect structural steel for well over 50 years in most rural environments. Even in harsher urban or coastal areas, you can generally expect two decades or more of solid performance.
- Consistent and Predictable Results: Galvanizing is done according to strict standards (like the Australian/New Zealand Standard 4680), so you know exactly what you’re getting. The thickness and quality of the coating are reliable, making performance easy to predict.
- Exceptionally Durable: Thanks to its unique metallurgical structure, a galvanized coating can stand up to rough treatment during transport, installation, and everyday use—it’s about as tough as protective finishes get.
- Self-Healing Qualities: If a galvanized surface gets scratched or nicked, the coating actually steps in to protect the exposed steel. It does this by corroding in place of the steel itself, so small damaged spots don’t need any touch-ups, unlike what you’d see with regular paint.
- All-Around Coverage: One of the big advantages of galvanizing is that it covers every part of the steel—corners, crevices, and all those hard-to-reach spots. Other coatings applied after assembly just can’t match this level of complete protection.
- Easy to Inspect: It’s straightforward to check a galvanized coating—most of the time, you can simply look at it, and there are simple, non-destructive tests for thickness if you want to be sure. If it looks good and continuous, you can trust that it is.
- Speeds Up Construction: Galvanized steel arrives on-site ready to use, so there’s no waiting around for surface prep, painting, or inspections. As soon as assembly is done, you can move right on to the next stage of construction or put the structure straight to work.
- Quick Application, No Weather Delays: The whole galvanizing process only takes a matter of minutes, and—unlike many coatings—there’s no need to worry about the weather slowing things down.
FAQs
What is the galvanization process?
Galvanization or galvanizing (also spelled galvanisation or galvanising) is the process of applying a protective zinc coating to steel or iron, to prevent rusting. The most common method is hot-dip galvanizing, in which the parts are coated by submerging them in a bath of hot, molten zinc.
Why is zinc used for galvanisation?
The reason that the galvanizing process uses zinc instead of other metals is that zinc oxidizes and experiences acid corrosion “sacrificially” to steel. That means that when zinc is in contact with steel, oxygen and acids will attack the zinc rather than the steel beneath it.
What is galvanization and its advantages?
Galvanizing, or galvanization, is a manufacturing process where a coating of zinc is applied to steel or iron to offer protection and prevent rusting. There are several galvanizing processes available, but the most commonly offered and used method is called hot-dip galvanizing.
Does galvanization need electricity?
Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. The process involves electroplating, running a current of electricity through a saline/zinc solution with a zinc anode and steel conductor.
What’s better zinc or galvanized?
Both zinc plating and galvanizing is an application of zinc plating. The big difference is thickness: zinc plating is normally 0.2 mils thick. Hot dip galvanizing might be 1.0 mil thick – you get over 5 times the protection with galvanizing.
Does galvanized steel rust?
Galvanized steel takes a long time to rust, but it will eventually rust. This type is carbon steel that has been galvanized or coated with a thin layer of zinc. The zinc acts as a barrier preventing oxygen and water from reaching the steel, providing advanced corrosion protection.